Plants and Darwinism
The limitations of natural selection
Editorial note (2014): Research since 1987 has fleshed out many of the biochemical details relating to this article, which reinforce the arguments put here.
Few people recognise plants as dynamic, complicated machines. Yet my own initial convictions of deliberate design arose from an appreciation of the vast biochemical and structural complexity of plant cells. Consideration of the elaborate mechanisms of certain specialised plants helped to strengthen my already strong impressions. I shall illustrate these, firstly, with two examples of plants showing movements before considering recognition phenomena as additional pointers towards deliberate design.
The fallacy of random mutations and Darwinian natural selection, as a cause for the origin of complexity, will also be discussed.
Throughout this article it will be taken for granted that natural selection is an acceptable process for the selection of phenotypes, and is in operation today as has been widely demonstrated. Natural selection is the process whereby either the environment or predators, or other factors, ‘weed out’ the less ‘fit’ individuals of a species (and hence their potential progeny) in much the same way as an examination weeds out the less capable students.
Plant movements and Darwinism
In the carnivorous plants, Utricularia (the bladderworts), small bladders of elaborate design are maintained under negative hydrostatic pressures of about 17kPa and possess an opening covered by a watertight trapdoor.1 The bladders are attached to underwater stems and when a tiny animal disturbs the hairs projecting from the trapdoor, the trapdoor suddenly swings inwards, and water, together with prey, is sucked into the bladder. The trapdoor then shuts tight; the entire process taking 0.03 seconds. Small worms and even young tadpoles have been observed to be sucked in and digested. There is strong evidence that the hairs of the trapdoor function as mechanoreceptors converting the mechanical stimulus to an electrical pulse which is transmitted to specialised cells activating the trapdoor. A collection of excellent photographs and diagrams of the mechanism can be found in Adrian Slack’s book Carnivorous Plants.2
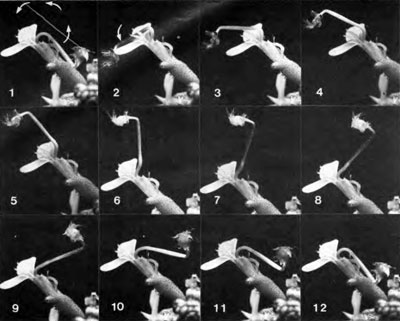
Very fast movements also occur in the flowers of the common trigger plant, Stylidium graminifolium. At Eaglehawk Neck in Tasmania, Australia, individual flowers reach one centimetre in size. The fast movement is a mechanism for cross-pollination, and is activated by cells sensitive to the probing of nectar-seeking flying insects. As shown in the series of photographs, when stimulated in this manner the column fires forward and upward in a fraction of second, depositing pollen on the back of the startled insect (not shown) sitting on the flower. The flower can also pick up non-self pollen in this way. Frame 1 shows the set column, and frame 2 its position immediately after firing. The large bushy head of the column is the pollen-containing structure (the anther), as well as the pollen-receiving structure (the stigma). They are fused in this species. After firing, the column completely resets itself within eight minutes (frames 2–12), and after a rest period of about 20 minutes it is ready to fire again. Resetting depends on an expenditure of metabolic energy by the tissues of the column. The intricate cellular events proposed by us,3 and later thoroughly confirmed and extended,4 could not have been predicted from traditional osmotic theory. The biophysics of the rest period presents a mystery as well.
Neither the movements in Utricularia nor those in Stylidium graminifolium find satisfactory evolutionary explanations with regard to the origin of such species. I would grade as illogical any hypothesis based on blind, chance mutations and natural selection.
Yet this is the philosophy invoked by neo-Darwinism. Dr Barbara Pickard, a well-known American biophysicist, states in her preface to a 1966 reprint edition of Charles Darwin’s book, The Power of Movement in Plants:
“This brings us to an indirect route to the main theme of ‘The Power of Movement in Plants’. It was a desire to find an evolutionary relationship between the many types of plant movements that motivated Darwin’s researches. Throughout the book he reiterated his beliefs that pronounced movements in response to specific stimuli could not have evolved in so many organs unless they were derived from a more trivial but almost universal tendency of all plant organs to carry out tiny movements.”5
This desire was based on his gradualistic belief as expressed in The Origin of Species.
“If it could be demonstrated that any complex organ existed which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down.”6
William Paley’s Natural Theology,7 written half a century before The Origin of Species and prescribed reading in British universities for more than a 100 years (a book which Darwin himself first loved and then rejected), argued for an opposite view. He reasoned logically “that we are capable both of observing certain characteristics in things and, by an experientially well-grounded analogy between like causes and like effects, of recognizing them as the unfailing marks of design. The most important of these marks are the co-operation of the parts and the resulting project of an end”. He felt that wherever we find these phenomena we may reasonably infer that they are the product of deliberate design; i.e. we may rationally also assert the existence of a designer. Paley emphatically dismissed the notion of random chance as a cause of complexity, and so do many of us today.

Recognition phenomena: further evidence for design in plants
One of the most amazing features of plants which students learn in some detail at university is the great variety of co-ordinated events necessary during vegetative and sexual reproduction. I do not know whether it strikes the students as such, but if not it should. Reproductive cycles are predestined. They are anything but haphazard, seemingly proceeding along a well defined and precise plan of action. And why should they not? After all, it is all coded for in advance. Reproductive cycles are so regular and reproducible, generation after generation, that their characteristics can be used to delineate species within the algae and fungi for classification purposes. In the vascular or ‘higher’ plants the cycle is so uniform that it serves only as a very gross mode of classification.
a) Reproduction in a non-flowering plant
In the sexual reproduction of the motile unicellular freshwater alga Chlamydomonas reinhardii, depicted in Figure 2, it would be utterly futile for male and female cells to collide and stick to one another if the overall plan of the life cycle had not included in advance the subsequent need to induce the synthesis of cell wall degrading enzymes following adhesion of their flagellae (swimming filaments). The mating process is characterised by dissolution of their cell walls, the formation of special mating structures in both the male (+) and female (-) protoplasts, and the extension of a fertilisation tube from the male (+) which then proceeds to fuse at the site of the female mating structure.8 This allows their cytoplasms to mix and their nuclei to combine. The complexities in human reproduction and development immediately spring to mind. It is this kind of innate need for forward planning and the virtually miraculous co-ordination of molecular events which compels one to invoke deliberate design.
But what makes mating pairs want to stick together in the first place? It is certainly not coincidence but is genetically provided for by the triplet DNA code. There are several things to note concerning the adhesion of mating pairs in the genus Chlamydomonas:
- Vegetative cells, which look identical to the sexually active cells and which also have two flagellae, cannot adhere to one another even when the male (+) and female (-) strains of the same species are mixed together. Thus vegetative cells, if left in their undifferentiated vegetative form, would never be able to mate sexually. Evolution via meiosis would stop here.
- Vegetative cells, of both the male and female strains, have to be induced (e.g. by nutritional deficiency) to divide and form sexually active male and female cells respectively—a remarkable transformation of complexity in one step, somewhat akin to a leaf-chewing, earthbound, multilegged caterpillar being transformed into a nectar-sucking, flying butterfly with six legs (but not to the same degree of complexity, of course).
- Adhesion between mating pairs occurs either along the tip or along the entire length of their flagellae.
- Adhesion is very species specific, even though cells of different species look alike and all have the same number of flagellae. Thus (+) Chlamydomonas reinhardii will never adhere to (-) Chlamydomonas eugametos, and vice versa. Recognition is therefore not based on a simple system.
- In the laboratory it is possible to use tiny fragments of female flagellae (like a sandwich) to cause close adhesion between two male partners of the same species. Such homosexual unions do not trigger subsequent events necessary for cytoplasmic fusion (compared to homosexual unions in humans). The nature of the message and the responses incurred upon true sexual union is still a mystery deserving of further research.
- Experiments have demonstrated that recognition between compatible flagellae involves complex glycoproteins9—protein molecules characterised by possessing side chains of stereo-specific sugars such as mannose, galactose, etc. Because of their partner specificity, compatible flagellae can obviously be pictured as having sites capable of ‘interlocking’ with one another in much the same way as two adjoining pieces of a jigsaw puzzle. In Chlamydomonas eugametos brief treatment of cells with enzymes which chew up proteins (e.g. trypsin), only affect the adhesion properties of the (-) sex cells thus providing evidence that the vital interlocking component on these flagellae involves proteins. Male (+) cells are not impaired when treated with the same enzymes. The reverse situation is true when a sugar-destroying enzyme (α-mannosidase) is used to pre-treat the mating strains before mixing. Only the adhesion capacity of the (+) cells can be affected with α-mannosidase. Thus adhesion between (-) and (+) cells in this species depends on the genetic code providing highly specific protein and sugar moieties on the flagellae of the (-) and (+) strains respectively, but only when the cells are sexually differentiated.
- The above indicate a high degree of order and purpose, once again implying thoughtfulness on the part of the designer.

b) Reproduction in flowering plants
Pollination and fertilisation is, no doubt, an already familiar subject to the reader, but what I want to emphasise here once again in considering this topic, is the role of recognition phenomena between male and female tissues or cells. Pollination is the transfer of male pollen from the anther of a flower to the female receptive tissue, the stigma. The pollen tube, formed on germination of the pollen grain, penetrates the tissues of the stigma and then grows down the style into the ovary as shown in Figure 3 (diagram not to scale). After entering the ovary the pollen tube grows towards the ovule which contains the egg nucleus.
When the pollen tube enters the structure known as the embryo sac, the tube discharges the two sperm nuclei which it carried down from the pollen grain, a distance of 0.2-10 cm depending on the species. The style in hibiscus is very long, as most gardeners will have observed. Provided all of these steps have been allowed to proceed without impediment, one of the sperm nuclei will fuse with the egg to form the zygote, which will develop into the new embryo.
Gross genetic boundaries are maintained by rejection of foreign pollen grains. To prevent inbreeding, which if excessive can be harmful,10 more than half the flowering plant families have specific mechanisms whereby an individual of a species will reject its own pollen and only accept pollen from a neighbour of the same species, i.e. within the species individuals are self-incompatible, but cross-compatible. Clover is a particularly good example of self-incompatible system. Every individual plant in a field of clover is self-incompatible. According to Lewis, the degree of efficiency of this system is so high that only about one in 22,000 pairs will not cross-pollinate.11 Other commonly cultivated plants such as sweet cherries, plums, pears, cabbage, Brussels sprouts and fodder kale also exhibit self-incompatibility. There will be varietal differences, of course.
In some orchard crops, and in cabbage, an entire variety may be self-incompatible and need to be crossed with another variety for seed (or fruit) production. This is why one finds alternate rows of different varieties of trees in old orchards. Modern varieties avoid such problems with only the flowers on the same tree being incompatible with respect to each other, but compatible with flowers on neighbouring trees. This is why bees are an important component of orchards in helping to spread pollen from one tree to the next. Orchardists in Florida are worried about the likely dramatic drop in crop yields should they be forced to use insecticides on African ‘killer’ bees predicted to invade the southern United States from Brazil (where 26 queens escaped from experimental hives in 1957).12
Research on compatible and incompatible pollination systems has boomed in recent years,13 at least in Melbourne, Australia, because of its potential impact on plant breeding, seed production, and the hopeful ‘creation’ of new species by crossing the set genetic boundaries. What has become clear in this research is that the obstacles which a pollen tube must overcome to reach the egg cell can be likened to an inspector attempting to reach the bars of gold bullion deep in Fort Knox. Apart from having to satisfy the initial ‘red-tape’, there will be numerous ‘gates’, each with its own key or password (all spelling deliberate design and intention).
Pollination is therefore the sum of a number of subprocesses each of which must be successfully completed to achieve fertilisation. As outlined in a previous work,14 the essential steps for fertilisation are:
- Pollen grains must hydrate on the stigma surface.
- The grains must germinate and form a pollen tube.
- Pollen tubes must continue to grow.
- Pollen tubes must penetrate the stigma surface and enter the style canal.
- Tubes have to grow all the way to the ovary.
- Tubes must enter the ovules.
- The tubes must be able to release the sperms.
- Finally, the sperms and egg cells must be compatible.
Abortion is the result of failure of any ONE of the sub-processes. The successful completion of each step dependents on elaborate molecular and physical signalling between the male and female cells. How would sexual reproduction in flowering plants have ever evolved by gradual haphazard processes?
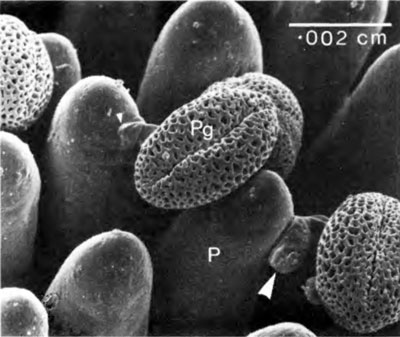
Incompatibility can be expressed in several ways. We shall look at two examples. The scanning electron micrograph (Figure 4) shows pollen grains of wild radish resting on the papillae of the stigma of their own flower. Rejection in this case has occurred at step 4. The pollen tube stops growing and forms a thickening of its wall at its point of contact with the papilla (arrow). The papilla shows a mutual response by also thickening its wall in this region. Figure 5 (upper) demonstrates that the chemical nature of the thickened wall is the same in both the pollen tube and the papilla (arrows). The material is callose which fluoresces a brilliant yellow when stained with aniline blue under the UV-fluorescence microscope. This type of rejection of self-pollen can be overcome in practice by flooding the stigma with high concentrations of carbon dioxide. Although the mechanism of formation of callose pads is not understood, it must involve recognition sites of some sort.
In contrast, Figure 5 (lower) shows a compatible pollen tube proceeding to grow down the stylar canal. In this photograph yellow autofluorescence from lignin in vascular bundles was suppressed using the periodic acid/Schiff reaction.15 The remaining yellow, UV-induced fluorescence indicates callose (a β1→3 polymer of glucose, distinct from cellulose). The tip of the pollen tube (right arrow) contains callose, as do portions of the pollen tube left behind (towards the left arrow). Moderate depositions of callose in growing pollen tubes is normal as they serve to seal the pollen tube at fairly regular intervals to conserve the volume of cytoplasm travelling forward with the tip. This in itself must be a carefully regulated system surely speaking of design—any flaws here and the cytoplasm would be dispersed or not allowed to grow forward.
A second type of abortion occurs in sweet cherries where the plants discriminate between self and non-self by the sudden arrest of growth of self-pollen tubes about half-way down the female tissues of the style. This so-called gametophytic system prevents inbreeding by having specific self-incompatibility genes (S genes) at a single nuclear gene locus with multiple alleles. The gene product is a glycoprotein (sugar-containing protein) whose localisation in the style has been correlated with the position of arrest of self-pollen tube growth. Recognition here, as in the Chlamydomonas system, appears to involve interactions between proteins and specific sugar residues in the pollen tubes and somewhere in the mucilage of the stylar canal.
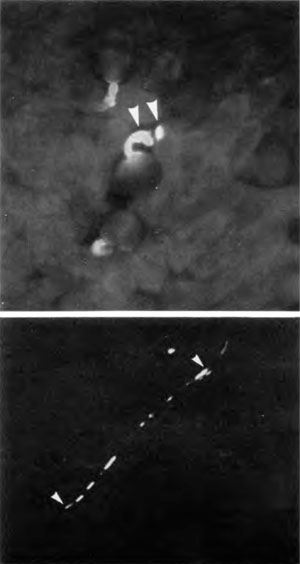
Upper: Incompatible self-pollination reaction on the stigma.
Lower: Apparently compatible self-pollination with successful growth of a self-pollen tube down the style.
Naturally, there are a host of intriguing questions still unanswered. What leads the pollen tubes specifically into the ovaries and then into the ovules? Calcium gradients may be involved here. What is it that prevents discharge of the sperm nuclei from the pollen tube prior to reaching the embryo sac? This is a pertinent question because in some species self-rejection is the premature release of sperm nuclei into the stylar canal. Whatever the final answers will be to these questions it seems a travesty to common sense to ascribe these wonderful mechanisms to chance products of random mutations during the course of evolution. It seems abundantly clear to me that there never was an evolution such as proposed by neo-Darwinism. Microevolution, as defined by us, has been well documented and verified however.
Conclusions and general comments on design and evolution
In an excellent article on design, Professor Kurt Marquart wrote: “You don’t need the Bible to prove there is a God.”16 The evidence of purposeful intention and design is all too clear. All biologists admit ‘apparent’ design and marvel that chance could have produced such a thing, but my understanding of science places science in complete harmony with the Bible. Gross genetic boundaries between different kinds of plants were established by God in Genesis 1:11-12 and the examples above have shown why this is so. If man chooses to change genetic boundaries by direct manipulations of the code itself, that by no means takes away from the truth of genetic boundaries in nature. Nature declares an infallible message even as the apostle Paul once said, “For the invisible things of him from the creation of the world are clearly seen, being understood by the things that are made [His handiworks] … so that they are without excuse” (Romans 1:20).
Charles Darwin said: “ … I gradually came to disbelieve in Christianity as a divine revelation … The old argument from design in nature, as given by Paley, which formerly seemed to me so conclusive, fails, now that the law of natural selection has been discovered.’17 In contrast to Darwin’s belief, and that taught in Australian universities today, natural selection will not really explain how complex organisms arose in the first place. Evolutionists themselves are questioning all kinds of Darwinian dogma.18,19
In the Genesis of Species, St George Mivart (1871) wrote: “How are we to account for the development of complex structures, like the wings of birds and bats, or the eyes of numerous animals? No doubt, once formed, these structures are very useful to their possessors. But during stages of early evolution, what advantages could they possibly have?”20 Henry Morris concluded: “There would be no reason at all for natural selection to favour an incipient wing or incipient eye or any other incipient feature.”20
Evolutionists have no rational answer to such questions except a blind faith which says that they are here so they must have evolved. In a report on vision research published in 1985, M.L. Considine writes: “So we can think of the eye as a precision optical system with an array of light-sensitive diodes functioning as image detectors: neural processing would have its parallel in microelectronics … but how did the lens evolve in the first place? Dr Sand’s model can’t answer that question: they simply show that one is needed.”21
Let us recapitulate what natural selection really means. Taking critical features such as reproductive cycles, the brain, muscle co-ordination, the kidney, the heart, the nervous systems, etc, it is only logical that natural selection would weed out at a greater rate those individuals in a population whose systems are either malfunctioning, or incomplete, or inferior. Thus natural selection tends to determine the final composition of a population and its genetic make-up. What natural selection can never tell us is how the population came to have all these wonderful features. Natural selection can only select! This is the chief limitation of natural selection if we choose to ask how organisms became complex.
To arrive at an answer to this question we come to the crux of evolution theory—random chance, through random mutations. Can you, the reader, really believe that pure chance caused the assembly of a community of bees, or the eye, or carnivorous plants for that matter? That is indeed what modern science teaches today. In a science classroom at university one is not allowed to tell students that the brain is so complex because God designed it to be so. Modern science not only refutes that living things were once deliberately designed, and for a purpose, but also attempts to discredit anyone who believes in a Creator God!22 I, for one, have been forbidden in my lectures either to point out weaknesses in evolution theory or provide evidence in support of a mature creation. Other lecturers, however, are free to disseminate all sorts of speculative evolutionary theories, as long as they say that they are speculative, even though their theories are in constant need of revision as more and more flaws come to light. Such is science and academic freedom.22
It is a tragedy that so many Christians have been mesmerised into security by denying their God in public.23 Such people comfortably coexist with evolutionists, parroting evolutionary concepts and language without ruffling feathers, welcoming the subtle back-slapping ‘Now here is a sensible fellow.’
References and notes
- Hill, B.S. and Findlay, G.P., The Power of Movement in Plants: The Role of Osmotic Machines, Quarterly Reviews of Biophysics 14(2):173–222, 1981. Return to text.
- Slack, A., Carnivorous Plants, Doubleday Australia Pty Ltd, Sydney, 1983. Return to text.
- Findlay, G.P. and Pallaghy, C.K., Potassium Chloride in the Motor Tissue of Stylidium, Australian Journal of Plant Physiology 5(2):219–229, 1978. Return to text.
- Findlay, N. and Findlay, G.P., Movement of Potassium Ions in the Motor Tissue of Stylidium, Australian Journal of Plant Physiology 11(6):451–457, 1984. Return to text.
- Darwin, C., The Power of Movement in Plants (1881), Unabridged republication, Da Capo Press, New York, 1966. Return to text.
- Darwin, C., The Origin of Species (1872), 6th edition, Collier Books, New York, 1962, p.182. Return to text.
- Paley, W., Natural Theology, Selections from 1802 edition, F. Ferre (ed), The Bobbs-Merrill Co. Inc., Indianapolis, 1963; Clarke, M.L. Paley: Evidences for the Man, SPCK, London, 1974. Return to text.
- Lembi, C.A., Unicellular Chlorophytes, In: Phytoflagellates, Developments in Marine Botany, Vol.2, E.R. Cox (ed), Elsevier/North Holland, New York, 1980, pp. 1–59. Return to text.
- Heslop-Harrison, J., Cellular Recognition Systems in Plants, Studies in Biology no. 100, Edward Arnold, London, 1978. Return to text.
- O’Brien, S.J., Wildt, D.E. and Bush, M., The Cheetah in Genetic Peril, Scientific American 254(5):68–76, 1986. Return to text.
- Lewis, D., Sexual Incompatibility in Plants, Studies in Biology no.110, Edward Arnold, London, 1979. Return to text.
- Gore, R., Those Fiery Brazilian Bees, National Geographic 149(4):491–501, 1976. Return to text.
- Fincham, J., Self-incompatibility in Plants, Nature 321(6065):20, 1 May 1986. Return to text.
- Harvey, J. and Pallaghy, C.K., The Bible and Science, Acacia Press, Blackburn, 1985. Return to text.
- Feder, N. and O’Brien, T.P., Plant Microtechnique: Some Principles and New Methods, American Journal Botany 55(1):123–142, 1968; Smith, M.M. and McCully, M.E., Mild Temperature ‘Stress’ and Callose Synthesis, Planta 136(1):65–70, 1977. Return to text.
- Marquart, K., From Paley to Darwin, The Lutheran, 13 February 1984, pp.30-33. Return to text.
- Darwin, C., In: Life and Letters of Charles Darwin, vol. l, F. Darwin (ed.), John Murray, London, 1887, pp.308-309. Return to text.
- Ho Mae-Wan, Saunders, P. and Fox, S.A., A New Paradigm for Evolution, New Scientist 109(1497):41-43, 27 February 1986. Return to text.
- The Quiet Revolution of Evolution Theory, New Scientist 109(1493):6330, January 1986. Return to text.
- Quoted in Kitcher, P., Abusing Science: The Case Against Creationism, MIT Press, Massachusetts, 1982. Return to text.
- Considine, M.L., Spectrum: Eyeballing the Eyeball, Ecos 42:27–28, 1984. Return to text.
- Thornton, I.W.B., Parsons, P.A., Stone, B.A., Waid, J.S. and Wardrop, A.B., Freedom of Thought, Correspondence, Nature 321(6067):191, 15 May 1986; Pallaghy, C.K. How Big was the Ark? Correspondence. Nature 320(6057):9, 6 March 1986. Return to text.
- Pallaghy, C.K., Creationism Down Under, Correspondence, Nature 316(6025):184, 18 July 1985. Return to text.






Readers’ comments
Comments are automatically closed 14 days after publication.